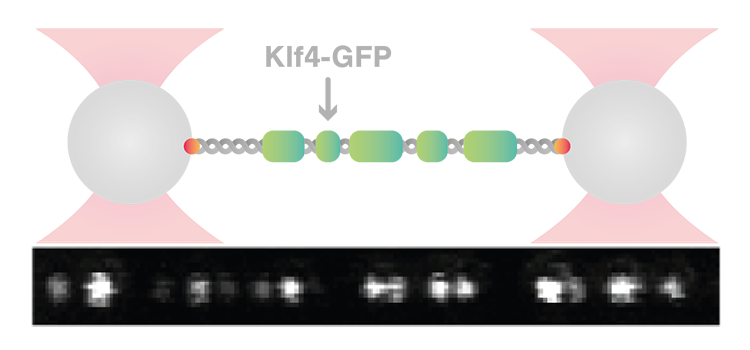
Researchers from the Max Planck Institute of Molecular Cell Biology and Genetics in Dresden, Germany, led by Stephan W. Grill, have shed light on the physical nature of the interaction between the pioneer transcription factor Klf4 (Krüppel-like factor 4) and DNA. Using the C-Trap®, which combines optical tweezers, confocal microscopy and controlled microfluidics, they were able to visualize how K1f4 binds to DNA in real time.
Pioneer transcription factors are crucial proteins for gene expression, as they can directly bind to DNA in its “closed” or condensed conformation making it accessible for other transcription factors to bind. K1f4 is a pioneer transcription factor involved in cell growth, differentiation and proliferation that presents a structured DNA-binding domain and a disordered activation domain. Previous studies have suggested that pioneer transcription factors form condensates – liquid-like separated compartments containing thousands of proteins – on DNA. However, the physics behind condensate formation and its relation to DNA sequence remains unknown.
Using the C-Trap, the scientists were able to hold a single λ-DNA molecule between two optically trapped beads and study its interaction with Klf4 at different concentrations. To track and quantify DNA binding in real time, they used Klf4 labeled with green fluorescent protein (GFP) and monitored the fluorescent signal using the confocal microscope that C-Trap is equipped with. These experiments showed that DNA drives condensation of K1f4 at lower concentrations than in solution, forming distinct foci. These foci were highly dynamic, as they could diffuse on DNA, grow in size within minutes, and even fuse. Furthermore, their number and intensity increased with protein concentration.
Notably, the C-Trap experiments revealed the existence of two types of K1f4 foci. Low protein concentrations resulted in dim foci accounting for the binding of single Klf4 molecules – an association referred to as the adsorbed state. As the concentration increased, the thin adsorbed state rapidly switched to a thick condensed state with more intense foci accounting for hundreds to thousands of proteins. The authors describe the condensation of K1F4 on DNA as a prewetting phenomenon, a precursor of wetting that happens at lower concentrations than those required for bulk phase separation. Further experiments demonstrated that the location of Klf4 condensates on DNA is sequence-dependent, a property that is essential for the regulatory role of transcription factors.
This work, which provides direct evidence on the interaction modes of pioneer transcription factors with DNA and offers mechanistic insights into the very first steps of gene expression, showcases the unique value of the C-Trap for protein condensation studies. By combining the use of optical tweezers and microfluidics to control and manipulate DNA-protein interactions, and confocal microscopy to directly visualize and precisely quantify these interactions over time, the C-Trap embodies the missing link from your toolbox in the study of protein condensate dynamics in the presence of DNA.
For further information, read the full article “Sequence dependent surface condensation of a pioneer transcription factor on DNA”, published in Nature Physics.
Are you interested in using dynamic single-molecule tools like the C-Trap for your research? Feel free to contact us for more information, a demo, or a quote.