We were happy to find yet another publication from the lab of Dr. Priya Banerjee with results from C-Trap® analyses! This time his lab examined the formation and features of vesicle-like RNA–protein structures, which differ from the more known uniform droplets. Apart from offering new insights to an emerging field, the results may impact future applications of RNA–protein structures, including pharmacological delivery systems.
In a multidisciplinary study implementing a range of analytical tools, the researchers identified characteristics of vesicular condensates that are distinct from the commonly studied RNA–protein droplets. They were able to induce and assess the reversible transition between isotropic droplets and anisotropic condensates dependent on RNA or protein ratios.
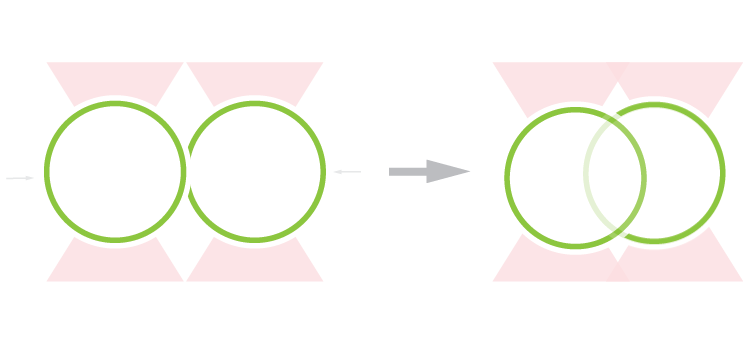
The C-Trap with correlated optical tweezers and fluorescence microscopy allowed the researchers to investigate the fusion properties of two vesicle-like condensates through controlled fusion. They found that the two structures initially form compartments, consisting of their respective rims and hollow spaces, to finally relax and create a single vesicle.
In a follow-up experiment based on phase transitions at different RNA/protein ratios, Alshareedah et al. examined the effect of RNA influx on droplet-to-vesicle transitions using the C-Trap’s microfluidics system. In short, they transferred optically trapped isotropic droplets to a microfluidic channel and induced a flow of RNA. They found that increased RNA stimulated a droplet-to-vesicle transition that could be readily reversed in the presence of RNase.
Their findings elegantly show how RNA–protein complexes assemble into vesicle-like structures – similar to lipids – that can transition to droplets depending on the presence of RNAs or proteins. This highlights little known properties of similar compounds, which can eventually be implemented for therapeutic purposes.
“[…] protein–RNA vesicles can be utilized as stimuli-responsive lipid-free cargo delivery systems for biotechnological applications (e.g., drug/gene delivery, insecticide/pesticide release),” the authors conclude. “These cargo delivery systems can be formulated carrier-free by directly utilizing proteins or nucleic acids of interest, and therefore achieve a high degree of target loading.”
Congratulations to Dr. Priya Banerjee at University at Buffalo, his lab, and all the authors involved in this fascinating study!
For more information, read the full article published in the journal PNAS titled “Phase transition of RNA−protein complexes into ordered hollow condensates”.
Are you interested in using dynamic single-molecule tools like the C-Trap® for your research? Feel free to contact us for more information, a demo, or a quote.