A recent publication by Losito et al. from the lab of Prof. David Rueda (Imperial College London), reveals new information on the kinetics and mechanism of Cas12a-DNA interaction. Using the C-Trap® the authors could observe in real-time Cas12a binding, target-search, and DNA cleavage under different conditions and hence expand the understanding of this enzyme specificity.
In the past years, CRISPR gene editing has emerged as a highly popular technology with a special focus on the variant Cas9. However, Cas12a has recently surfaced as an excellent alternative that shows less off-target effects in cells compared to Cas9. But despite several studies on the structure and function of Cas12a, mechanistic understanding of this variant remains incomplete.
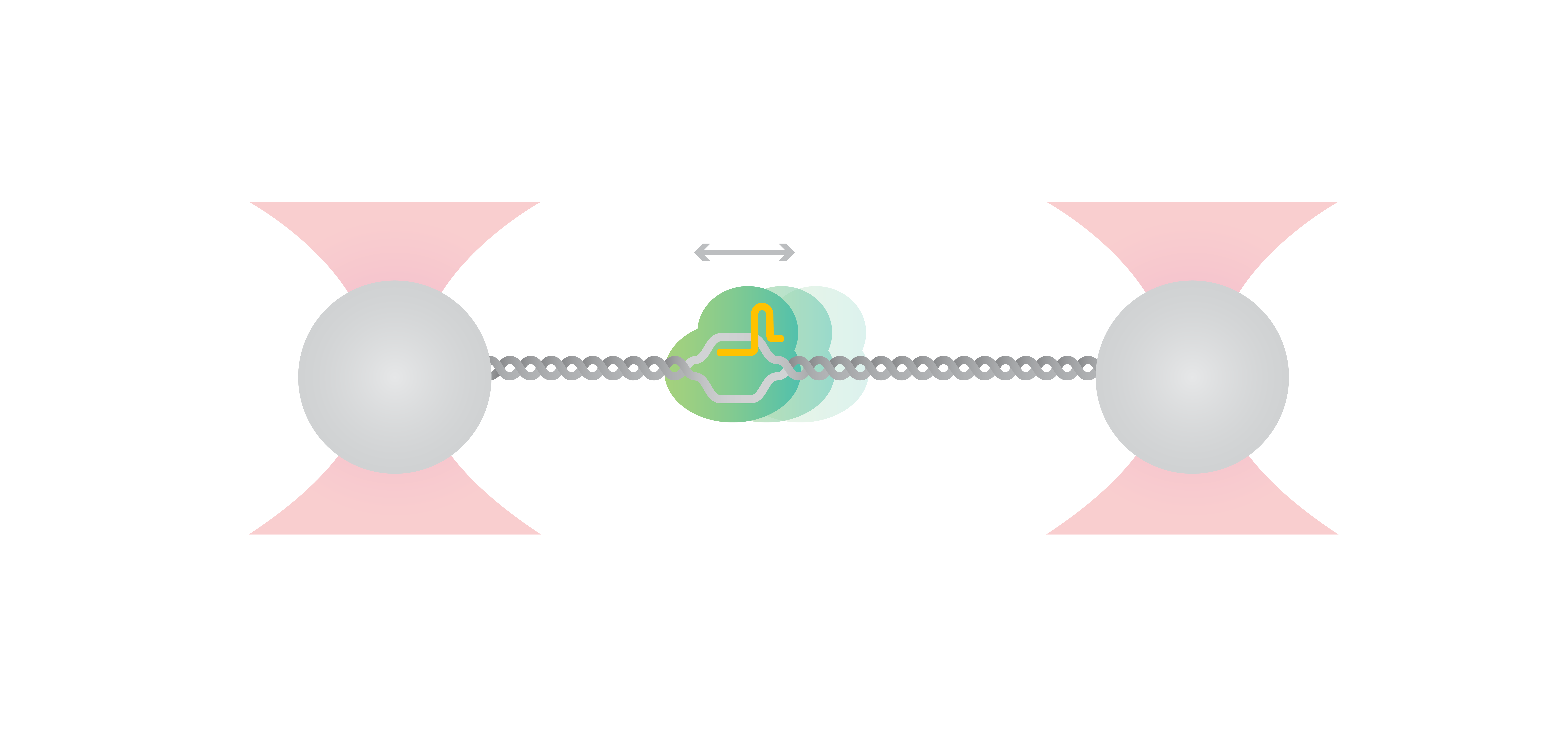
In this article, the researchers employed the C-Trap to further investigate the mechanism of target search and cleavage by Cas12a on stretched λ-DNA. For the experimental set-up, biotinylated λ-DNA was tethered between two optically trapped beads while the activity of fluorescently-labeled Cas12a complexes could be simultaneously visualized by confocal microscopy.
Their investigation reveals that Cas12a complex binds to DNA away from their target. Subsequently, it diffuses randomly on the DNA with an average speed of 1 kbp/s, moving in both directions, until it finds and stably binds its target.
After target-binding, DNA cleavage occurs within seconds when DNA is held at its contour length. However, when stretching the DNA the cleavage is increasingly hindered. Their data suggests a distance to transition state of x = 1.8 ± 0.3 nm, which indicates a rate-limiting conformational change between DNA binding and cleavage. Interestingly, this value corresponds to the ~ 2 nm distance – found in several structural studies – that the Rec domain moves to bring the target strand towards the cleavage site in the RuvC nuclease. These results are an excellent example of how dynamic single-molecule methods provide direct proof of molecular interactions and become indispensable in validating structural findings.
Their results also showed that off-target binding increases with higher forces ( > 20 pN) as known for Cas9, but that cleavage itself is inhibited for those binding events. As force stretching results in DNA melting and R-loop formation, these findings implicate that potential off-target events in negatively supercoiled DNA inside cells may also not be cleaved.
In summary, this publication provides deeper insights into the mechanistic behavior of Cas12a complexes on double-stranded DNA that are crucial for our understanding and further development of CRISPR gene editing applications. To read the full article titled “Cas12a target search and cleavage on force-stretched DNA” visit Physical Chemistry Chemical Physics.