In a recent publication by Keenen et al. in eLife, the authors uncover the biophysical properties of heterochromatin protein 1α (HP1α) and present a new model on how it mediates DNA condensation and phase separation. Employing a combination of ensemble and single-molecule assays, they found that within phase-separated HP1α-DNA condensates, HP1α behaves like a dynamic liquid while compacted DNA molecules are locally constrained. Using the C-Trap®, enabled insights into how these complexes might function inside the cell, revealing that the condensates are highly resistant to large forces but relax at sustained forces.
Eukaryotic heterochromatin is a tightly packed form of DNA that ensures the suppression of transcription in the respective regions. Particularly, the interaction with proteins from the HP1 family results in highly conserved heterochromatin forms. Despite research in this field, questions remained on how the highly dynamic HP1 molecules enable long-term stable chromatin states and maintain compacted DNA at relatively high forces. The valuable combination of dynamic single-molecule techniques with bulk experiments sheds light on these questions. The unique ability of the C-Trap allowing to visualize interactions while applying tension on the DNA and measuring its response at the same time contributed to the understanding of the properties of HP1α-DNA condensates at biologically relevant forces.
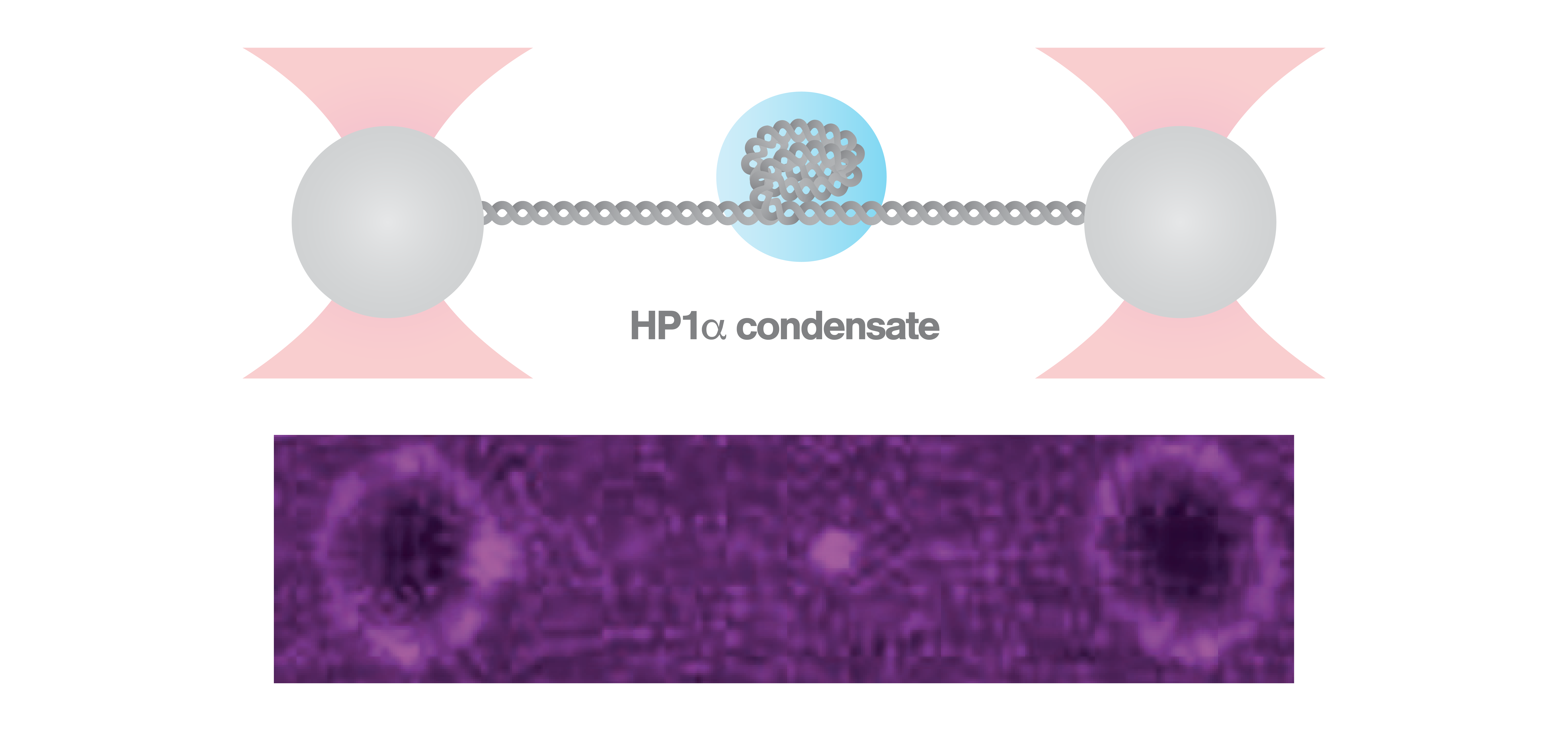
To form HP1α-DNA structures, the scientists used C-Trap optical tweezers correlated with confocal microscopy to trap a DNA molecule and expose it to fluorescently labeled HP1α. The formation of a fluorescent HP1α-DNA condensate was completed within 30 sec as observed via confocal fluorescence microscopy. Applying repetitive stretching and relaxing of the DNA, as well as continuous stretching, at low (25 pN) and high (40 pN) forces, the scientists discovered that the HP1α-DNA condensates respond by resisting and strengthening after instantaneous forces, but were relaxing and weakening after sustained forces.
Specifically, the findings from the C-Trap experiments indicate how heterochromatin confers mechanical stability within cells during events like chromosome segregation or mechano-chemical signaling at the nuclear membrane, as well as the ability of HP1α-mediated heterochromatin to adjust to high cellular forces by relaxation. These results highly contribute to our understanding of the biophysical properties of chromatin compaction.
Learn more about the data acquired by the C-Trap and further results, by reading the full article titled “HP1 proteins compact DNA into mechanically and positionally stable phase-separated domains” that is published in eLife.