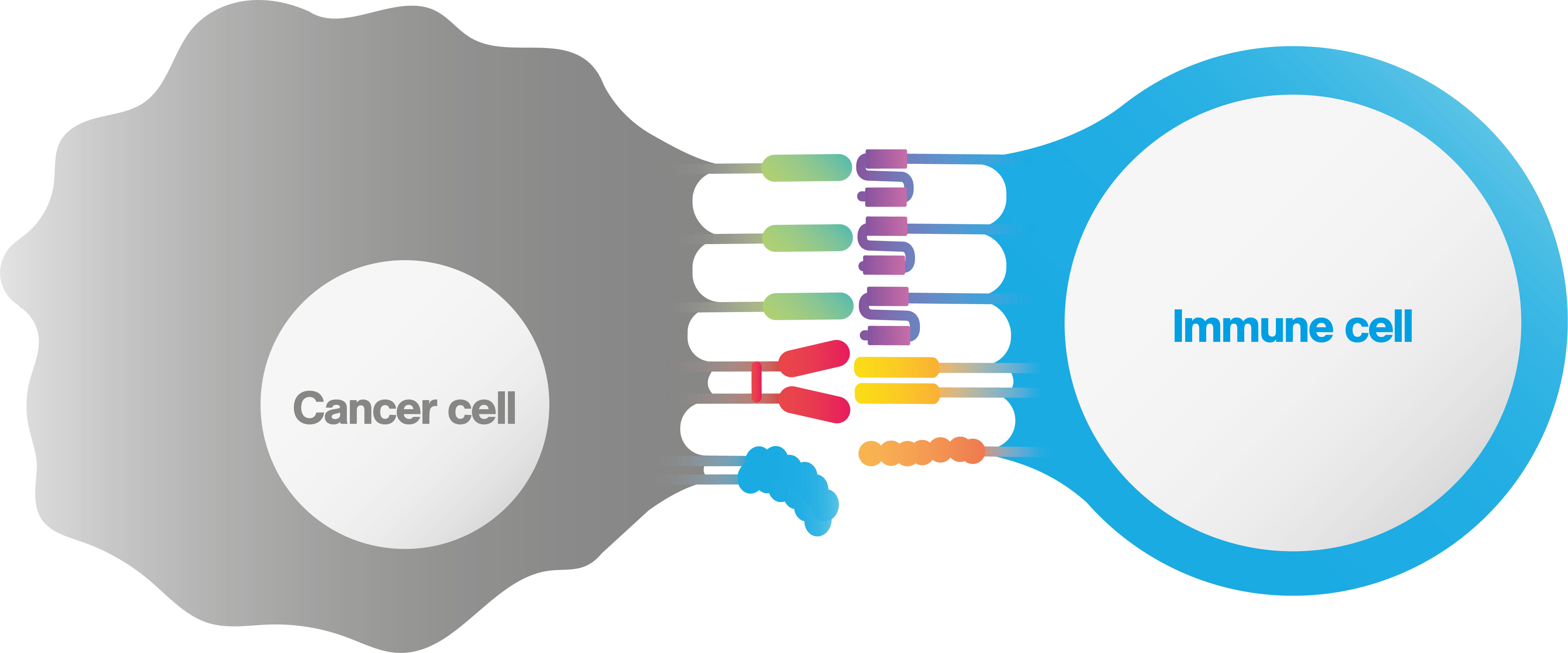
What is an immune synapse?
An immunological (or immune) synapse is the interface between a cancer and an immune cell , which is formed in a highly stable, organized manner. The immunological synapse is composed of all intercellular interactions taking place at the interface between the interacting pair, including TCR clustering, co-receptor binding, cell-cell adhesion proteins and even orientations and valencies (figure 1).
Proper formation of the immune synaptic interface is required to initiate immune cell activation, achieve sustained proliferation and increase cytokine secretion.
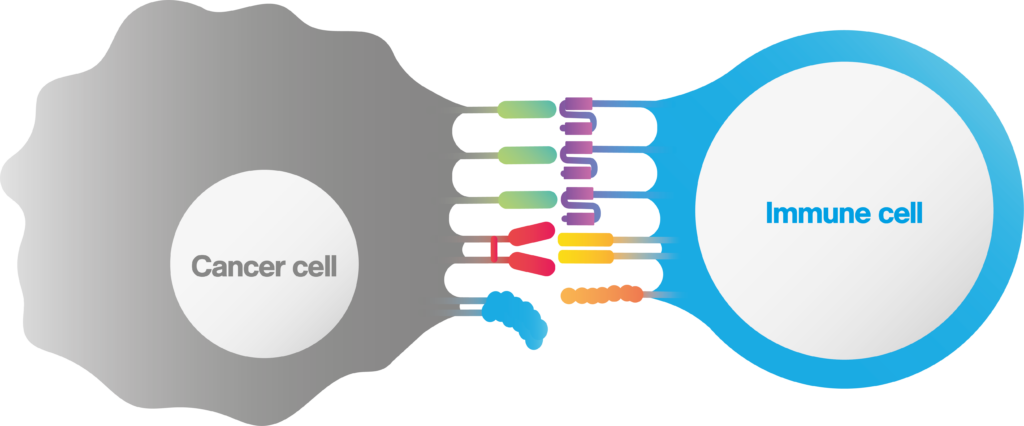
Different immunological synapse formation
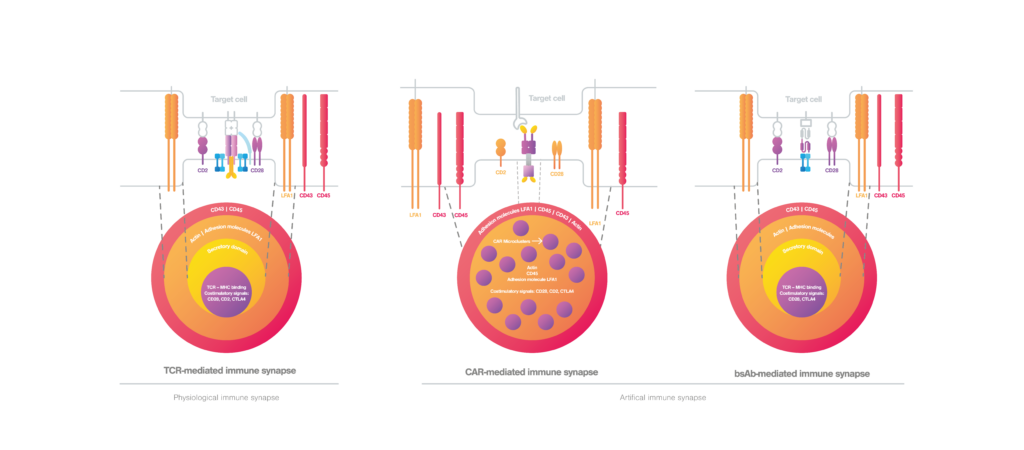
Immunological synapse formation mediated by T cells
Under physiological conditions where the cancer cells are recognized by T cells, T cell movement is suppressed and immunological synapse formation starts upon TCR engagement (Alcover et al., 2016). In a mature TCR-mediated immunological synapse, the endosomal compartment, cytoskeleton, and signaling network are finely tuned to achieve proper T cell activation and effective immune responses (Soares et al., 2013). A proper TCR-mediated immunological synapse displays a well-organized bullseye structure (figure 2, left), where the cytoskeleton filaments mediate the centripetal movement of TCR microclusters to achieve an adequate T cell activation (Martín-Cófreces et al., 2014). During T cell effector functions, lytic granules or cytokines are released to the synaptic cleft by cytotoxic or helper T cells, respectively (Huse et al., 2006; Stinchcombe et al., 2006). For a sustained T cell activation and secured cell-cell adhesion, the immunological synapse requires actin clearance, polarization of the microtubule fibbers as well as integrin activity (Martín-Cófreces et al., 2018).
Immunological synapse formed in engineered T cells
When it comes to engineered T cells, the situation can be different. While the classical bullseye structure is maintained in bsAb-mediated synapse, CAR-mediated synapse displays a nonclassical structure. Here, the actin cytoskeleton is not completely cleared from the center, and CAR microclusters and signaling molecules are dispersed at the interface (figure 2, middle) (Davenport et al., 2018; Watanabe et al., 2018). In the CAR-mediated immunological synapse, the lytic granule secretion is rapidly triggered upon CAR engagement as an immediate response to antigen recognition even before the microtubule polarization takes place. Compared to conventional TCR-mediated immunological synapse, CAR-mediated immunological synapse drives rapid cytotoxicity, but has shorter activating signals and shorter cell-cell interactions that may therefore reduce cell persistence (Bertrand et al., 2013).
Why Tuning Synaptic Interactions and Strength Holds the Key to Cutting-Edge Cell Therapy Designs
The immune synapse serves as a pivotal interface between immune cells and cancer cells, enabling signal communication and orchestrated responses crucial in immune function. The impact of synaptic tuning on bolstering CAR design is evidenced in a Nature paper by Chockley et al.. (2023). The implementation of synapse-tuned CAR immune cells demonstrates a remarkable surge in synaptic strength, leading to an increased cytokine secretion, an elevated tumor killing, and a prolonged survival across different in vivo tumor models, including solid tumors. Skokos et al. and Staufer et al. also showed that strengthening the immune synapse formation in BsAbs improved binding and reduced tumor burden. Such findings indicate that understanding the synaptic formation becomes paramount in deciphering cell-cell interactions, immune surveillance, and its role in cancers.
A solution for quantifying the overall synaptic strength is known as cell avidity analysis. By integrating cell avidity into preclinical assessments, researchers gain valuable insights into the intricate biological nuances inherently tied to immune cell functionality. Furthermore, this approach aids in the early identification of efficacious immune cell candidates. This early screening not only enhances the prospects of clinical success but also proves cost-effective and time-saving by minimizing reliance on in vivo mouse models.
References
Alcover, A., Di Bartolo, V., & Roda-Navarro, P. (2016, December 21). Editorial: Molecular Dynamics at the Immunological Synapse. Frontiers in Immunology. https://doi.org/10.3389/fimmu.2016.00632
Soares, H., Henriques, R., Sachse, M., Ventimiglia, L. N., Alonso, M. A., Zimmer, C., Thoulouze, M. I., & Alcover, A. (2013, October 7). Regulated vesicle fusion generates signaling nanoterritories that control T cell activation at the immunological synapse. Journal of Experimental Medicine. https://doi.org/10.1084/jem.20130150
Martín-Cófreces, N. B., Baixauli, F., & Sánchez-Madrid, F. (2014, January 1). Immune synapse: conductor of orchestrated organelle movement. Trends in Cell Biology. https://doi.org/10.1016/j.tcb.2013.09.005
Huse, M., Lillemeier, B. F., Kuhns, M. S., Chen, D. S., & Davis, M. M. (2006, January 29). T cells use two directionally distinct pathways for cytokine secretion. Nature Immunology. https://doi.org/10.1038/ni1304
Stinchcombe, J. C., Majorovits, E., Bossi, G., Fuller, S. D., & Griffiths, G. M. (2006, September 28). Centrosome polarization delivers secretory granules to the immunological synapse. Nature. https://doi.org/10.1038/nature05071
Martín-Cófreces, N. B., & Sánchez-Madrid, F. (2018, May 30). Sailing to and Docking at the Immune Synapse: Role of Tubulin Dynamics and Molecular Motors. Frontiers in Immunology. https://doi.org/10.3389/fimmu.2018.01174
Davenport, A. J., Cross, R., Watson, K. A., Liao, Y. H., Shi, W., Prince, H. M., Beavis, P. A., Trapani, J. A., Kershaw, M. H., Ritchie, D., Darcy, P. K., Neeson, P. J., & Jenkins, M. R. (2018, February 12). Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proceedings of the National Academy of Sciences of the United States of America. https://doi.org/10.1073/pnas.1716266115
Watanabe, K., Kuramitsu, S., Posey, A. D., & June, C. H. (2018, October 26). Expanding the Therapeutic Window for CAR T Cell Therapy in Solid Tumors: The Knowns and Unknowns of CAR T Cell Biology. Frontiers in Immunology. https://doi.org/10.3389/fimmu.2018.02486
Bertrand, F., Müller, S., Roh, K. H., Laurent, C., Dupré, L., & Valitutti, S. (2013, March 27). An initial and rapid step of lytic granule secretion precedes microtubule organizing center polarization at the cytotoxic T lymphocyte/target cell synapse. Proceedings of the National Academy of Sciences, 110(15), 6073–6078. https://doi.org/10.1073/pnas.1218640110
Chockley, P., Ibañez-Vega, J., Krenciute, G., Talbot, L. J., & Gottschalk, S. (2023, February 2). Synapse-tuned CARs enhance immune cell anti-tumor activity. Nature Biotechnology. https://doi.org/10.1038/s41587-022-01650-2
Skokos, D., Waite, J. C., Haber, L., Crawford, A., Hermann, A., Ullman, E., Slim, R., Godin, S., Ajithdoss, D., Ye, X., Wang, B., Wu, Q., Ramos, I., Pawashe, A., Canova, L., Vazzana, K., Ram, P., Herlihy, E., Ahmed, H., . . . Yancopoulos, G. D. (2020, January 8). A class of costimulatory CD28-bispecific antibodies that enhance the antitumor activity of CD3-bispecific antibodies. Science Translational Medicine, 12(525). https://doi.org/10.1126/scitranslmed.aaw7888
Staufer, O., Leithner, A., Zhou, S., Crames, M., Comeau, S. R., Young, D., Low, S. H., Jenkins, E., Davis, S. J., Nixon, A. E., Pefaur, N. B., Kasturirangan, S., & Dustin, M. L. (2022, June 17). Segmental flexibility of bispecific T-cell engagers regulates the dynamics of immune synapse formation. bioRxiv (Cold Spring Harbor Laboratory). https://doi.org/10.1101/2022.06.15.496334